
M=moles of solute/liter of solution dilution water is added ML of solute/ mL of solution x 100% mass volume percent (m/v) g of solute/mL of solution x100% molarity moles of solute per volume of solution G of solute/g of solute + g of solvent x 100%= (m/m) volume percent (v/v) percent volume of solute tovolume os solution in mL

Have undissolved solute at the bnottom of the container solubility depends on _? temperature solids solubility _as temp increases increases gases solubility _as temp increases decreases henry's law the solubility of a gas in a liquid is dir3ctly related to the pressure of that gas above the liquid concentration of a solution equation amount of solute/amount of solution mass percent (m/m) concentration by mass in a solution
TEXTLAB ORGANIC CHEMISTRY CHAPTER 9 HOW TO
Provide your students with the side-by-side English/Spanish Glossary (Glossary/ Glosario)-a unique learning tool for ELL students.Weak electrolytes dissociate only slightly, forms a solution with few ions nonelectolytes dissolve as molecules in waterĭoes not produce ions in water and does not conduct an electrical current equivalents is the amount of a electrolyte or an ion that provides 1mole of electrical charge (mole x charge #) (mEq/L) how the concentration of electrolytes in fluids are expressed 1 Eq = _mEq/L 1000 How to tell the equivalents in moles it is the same as then ionic charge of the compound solubillity max amount of solute that dissolves in a specific amount of solvent are solutes temp sensitive yes expression for solubility g o solute/ 100 g water unsaturated solutions contain less that the max amount ofsoluteĬan dissolve more solute saturated solutions cantainn the max amount of solute.Offer a variety of review and practice opportunities with Example Problems, Practice Problems, and Supplemental Practice Problems.Help students prepare for local, state, and national tests with Standardized Test Practice and Test-Taking Tips in the chapter assessment.Engage your students with exciting, colorful introductions to the chapter content, including a Launch Lab, previews like What I Already Know and Reading Chemistry, and a link to Chemistry Online.Additional resource options are available for forensics, small-scale and probeware labs.
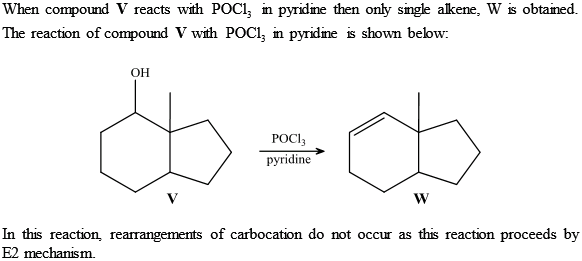
Provide clear chemistry application of key concepts with inquiry-based Launch Labs, safe and “doable” MiniLabs, and classroom-proven ChemLabs.
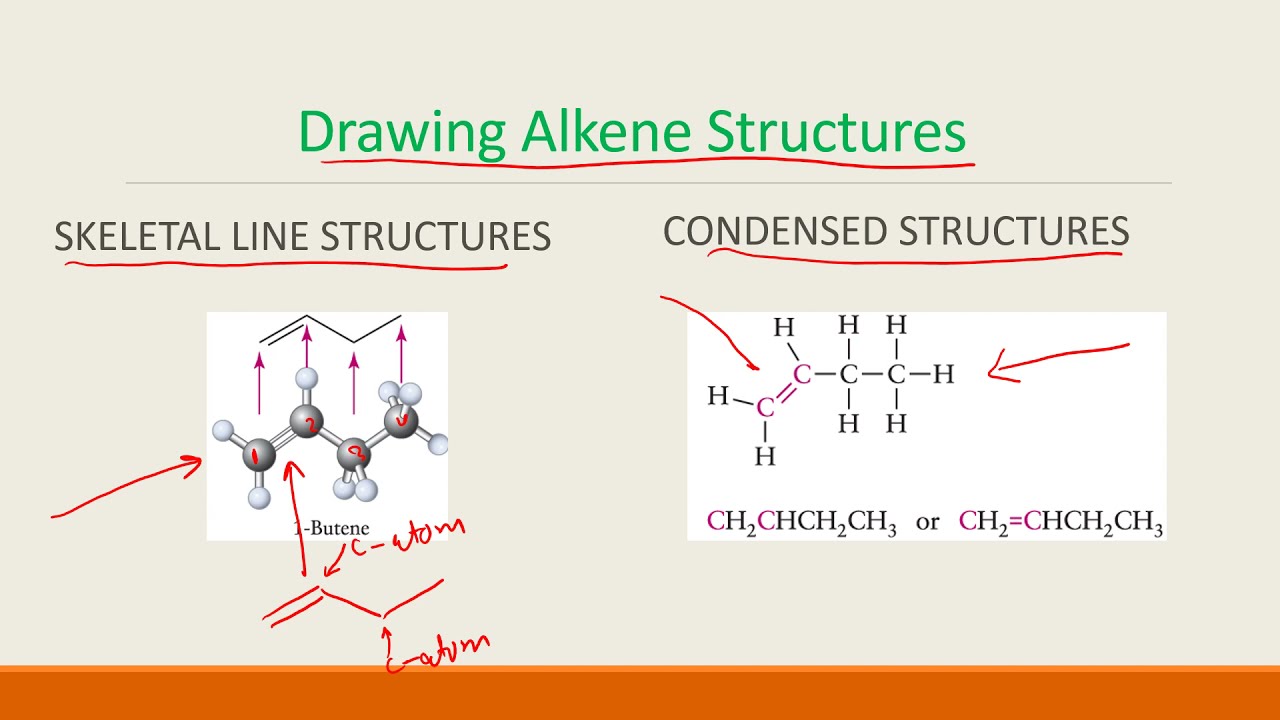
Using Chemistry: Concepts and Applications, you can:


 0 kommentar(er)
0 kommentar(er)
